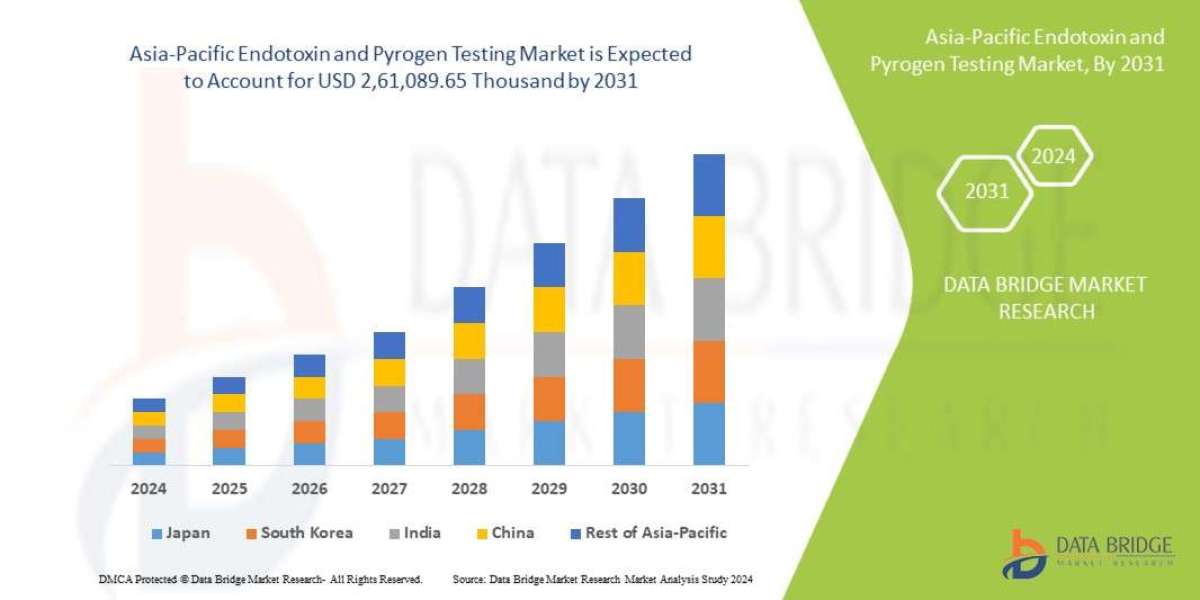
Executive Summary Asia-Pacific Endotoxin and Pyrogen Testing Market :
Data Bridge Market Research analyses that the Asia-Pacific endotoxin and pyrogen testing market which was USD 1,06,205.98 thousand in 2023, is expected to reach USD 2,61,089.65 thousand by 2031, and is expected to undergo a CAGR of 11.9% during the forecast period of 2024 to 2031.
Global Asia-Pacific Endotoxin and Pyrogen Testing Market report brings into light key market dynamics of the sector. The competitive landscape section of the report gives a clear insight into the market share analysis of key industry players. The company profiles of all the major market players and brands that are dominating the Asia-Pacific Endotoxin and Pyrogen Testing Market with moves like product launches, joint ventures, merges and accusations which in turn is affecting the sales, import, export, revenue and CAGR values have been cited in the report. The study consists of a market attractiveness analysis, wherein each segment is benchmarked based on its market size, growth rate, and general attractiveness.
The Asia-Pacific Endotoxin and Pyrogen Testing Market report is a professional yet exhaustive study on the current state for the market. The report is based on the market type, organization size, availability on-premises and the end-users’ organization type, and the availability in areas such as North America, South America, Europe, Asia-Pacific and Middle East & Africa. The market report also contains the drivers and restraints for the Asia-Pacific Endotoxin and Pyrogen Testing Market that are derived from SWOT analysis, and also shows what all the recent developments, product launches, joint ventures, mergers and acquisitions by the several key players and brands that are driving the market are by systemic company profiles. The company profiles of all the key players and brands that are dominating the Asia-Pacific Endotoxin and Pyrogen Testing Market have been taken into consideration here.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Asia-Pacific Endotoxin and Pyrogen Testing Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/asia-pacific-endotoxin-and-pyrogen-testing-market
Asia-Pacific Endotoxin and Pyrogen Testing Market Overview
**Segments**
- On the basis of product type, the Asia-Pacific Endotoxin and Pyrogen Testing Market can be segmented into consumables, instruments, services, and software. The consumables segment is expected to dominate the market due to the constant demand for reagents and kits in endotoxin and pyrogen testing. Instruments segment is anticipated to witness significant growth owing to advancements in technology and the introduction of innovative testing equipment. Services and software segments are also poised to experience steady growth as the need for efficient testing services and digital solutions increases in the region.
- By test type, the market can be categorized into LAL tests, in-vitro tests, and rabbit tests. The LAL tests segment is expected to hold the largest market share due to its widespread adoption in endotoxin and pyrogen testing. In-vitro tests are projected to witness significant growth as they offer reliable results without the need for animal testing. Rabbit tests are expected to have a smaller market share, but they are still utilized in some instances for their sensitivity in detecting endotoxins and pyrogens.
- Based on application, the Asia-Pacific Endotoxin and Pyrogen Testing Market can be segmented into pharmaceuticals, medical devices, biologics, and other applications. The pharmaceuticals segment is anticipated to lead the market as stringent regulations mandate endotoxin and pyrogen testing for drug approvals. The medical devices segment is expected to grow steadily due to the increasing focus on product safety and quality. Biologics and other applications segments are also poised for growth as the demand for endotoxin and pyrogen testing expands across various industries.
**Market Players**
- Some of the key players operating in the Asia-Pacific Endotoxin and Pyrogen Testing Market include Charles River, Lonza, Merck KGaA, Thermo Fisher Scientific, Associates of Cape Cod, Inc., GenScript, WuXi AppTec, Nelson Laboratories, and Pacific Biolabs among others. These companies are focusing on strategic partnerships, product developments, and expansions to enhance their market presence in the Asia-Pacific region. The increasing emphasis on regulatory compliance and quality control is driving the growth of these market players as they offer advanced endotoxin and pyrogen testing solutions to meet the evolving needs of the industry.
The Asia-Pacific Endotoxin and Pyrogen Testing Market is witnessing significant growth and evolution across various segments. One key aspect that could impact the market dynamics is the rising awareness and emphasis on product safety and quality in industries such as pharmaceuticals, medical devices, and biologics. As regulatory requirements become more stringent, the demand for reliable endotoxin and pyrogen testing solutions is expected to soar. This presents a lucrative opportunity for market players to develop advanced testing technologies that can meet the evolving needs of the industry.
In terms of product type segmentation, the consumables segment is poised to dominate the market due to the continuous demand for reagents and kits in endotoxin and pyrogen testing processes. This segment is likely to witness steady growth as testing requirements persist in various industries. Additionally, the instruments segment is anticipated to experience significant growth driven by technological advancements and the introduction of innovative testing equipment. As companies strive to enhance testing efficiency and accuracy, the demand for advanced instruments is expected to rise. Similarly, the services and software segments are also projected to grow as the industry shifts towards digital solutions and efficient testing services.
Considering the test type segmentation, the LAL tests segment is forecasted to maintain its dominance in the market due to widespread adoption and proven reliability in endotoxin and pyrogen testing. In-vitro tests are also expected to witness significant growth as they offer credible results without the need for animal testing, aligning with the growing emphasis on ethical and sustainable practices in testing processes. While the rabbit tests segment may have a smaller market share, their sensitivity in detecting endotoxins and pyrogens could still find application in specific instances where other tests may not be as effective.
On the application front, the pharmaceuticals segment is anticipated to lead the market due to regulatory mandates requiring endotoxin and pyrogen testing for drug approvals. The medical devices segment is also expected to grow steadily as the industry places a greater focus on product safety and quality assurance. The biologics and other applications segments are poised for growth as the demand for endotoxin and pyrogen testing expands across various industries beyond pharmaceuticals and medical devices. This diversification of applications could drive market growth and provide new opportunities for market players operating in the Asia-Pacific region.
In conclusion, the Asia-Pacific Endotoxin and Pyrogen Testing Market is witnessing robust growth driven by factors such as regulatory compliance, technological advancements, and increasing awareness about product safety. Market players are responding to these trends by focusing on strategic partnerships, product innovations, and expansions to strengthen their market presence. As the industry continues to evolve, the demand for reliable and efficient endotoxin and pyrogen testing solutions is expected to rise, presenting ample prospects for growth and innovation in the market.The Asia-Pacific Endotoxin and Pyrogen Testing Market is witnessing a shift towards greater emphasis on product safety and quality across various industries, particularly pharmaceuticals, medical devices, and biologics. With stringent regulations in place mandating endotoxin and pyrogen testing for drug approvals, the demand for reliable testing solutions is expected to drive market growth. This regulatory landscape presents an opportunity for market players to develop advanced technologies that can cater to the evolving needs of the industry and ensure compliance with quality standards.
Segmentation based on product type reveals that the consumables segment is anticipated to dominate the market due to the continuous demand for reagents and kits in testing processes. As industries continue to prioritize testing requirements, this segment is likely to experience steady growth. Furthermore, the instruments segment is expected to witness significant growth propelled by technological advancements and the introduction of innovative testing equipment. Companies are striving to enhance testing efficiency and accuracy, thereby driving the demand for advanced instruments in the market. Additionally, the services and software segments are projected to grow as the industry transitions towards digital solutions and more efficient testing services.
In terms of test type segmentation, LAL tests are forecasted to maintain their dominance in the market due to their widespread adoption and proven reliability in endotoxin and pyrogen testing. Meanwhile, in-vitro tests are expected to witness significant growth as they offer credible results without the need for animal testing, aligning with the industry's focus on ethical practices. Although the rabbit tests segment may have a smaller market share, their sensitivity in detecting endotoxins and pyrogens could still find application in specific instances where other tests may not be as effective.
The application segment highlights the pharmaceuticals industry as a leader in the market, driven by regulatory mandates necessitating endotoxin and pyrogen testing for drug approvals. Additionally, the medical devices segment is expected to grow steadily as companies place a stronger emphasis on product safety and quality assurance. The biologics and other applications segments are also poised for growth, reflecting the expanding demand for testing solutions across various industries beyond pharmaceuticals and medical devices. This diversification of applications could fuel market growth and offer new avenues for market players in the Asia-Pacific region to explore.
In conclusion, the Asia-Pacific Endotoxin and Pyrogen Testing Market is evolving in response to regulatory compliance measures, technological advancements, and a heightened focus on product safety. Market players are actively engaging in strategic partnerships, product innovations, and expansions to enhance their market presence and cater to the increasing demand for reliable testing solutions. As the industry evolves, the market is expected to witness significant growth, offering ample opportunities for innovation and growth in the Asia-Pacific region.
The Asia-Pacific Endotoxin and Pyrogen Testing Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/asia-pacific-endotoxin-and-pyrogen-testing-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Answers That the Report Acknowledges:
- Asia-Pacific Endotoxin and Pyrogen Testing Market size and growth rate during forecast period
- Key factors driving the Asia-Pacific Endotoxin and Pyrogen Testing Market
- Key market trends cracking up the growth of the Asia-Pacific Endotoxin and Pyrogen Testing Market.
- Challenges to Asia-Pacific Endotoxin and Pyrogen Testing Market growth
- Key vendors of Asia-Pacific Endotoxin and Pyrogen Testing Market
- Opportunities and threats faces by the existing vendors in Global Asia-Pacific Endotoxin and Pyrogen Testing Market
- Trending factors influencing the market in the geographical regions
- Strategic initiatives focusing the leading vendors
- PEST analysis of the Asia-Pacific Endotoxin and Pyrogen Testing Market in the five major regions
Browse More Reports:
Global End Stage Renal Disease (ESRD) Drug Market
Global Barley Flakes Market
Global Heavy Metal Testing in Food and Beverage Application Market
Korea E-commerce Packaging Market
Asia-Pacific Braze Alloys Market
Global Bioinformatics Market
Global Marine and Protective Coatings Resins Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- [email protected]